Pharma compliance teams are used to change. But 2025 tested the limits of what typical pharmacovigilance teams are resourced to handle, with policy shifts, regulatory crackdowns, and high-profile public claims dominating headlines in the US and beyond.
In recent weeks:
- The US federal government announced a 100% tariff on patented drug imports, with exemptions that introduced uncertainty and potential global ripple effects for pharmaceutical supply chains.
- The FDA issued 100 cease-and-desist letters as part of an enforcement wave targeting direct‑to‑consumer (DTC) advertising practices.
- A widely publicized claim linking Tylenol use in pregnancy to autism gained significant traction online, creating sustained reputational and financial pressure for parent company Kenvue.
- ACIP vaccine guidance shifted, introducing ambiguity into public health messaging and prompting heightened discussion around vaccination.
As political pressure, regulatory actions, and fast-moving public narratives converged, pharma teams saw how quickly online conversations can escalate into compliance and reputational risks.
From regulatory actions to shifts in public discourse, our analysis examines the developments that reshaped the pharma risk landscape in late 2025. We explore how these events exposed critical gaps in pharmacovigilance workflows, and why real-time risk detection is now a core requirement for pharma compliance and brand protection.
Adverse event detection under FDA scrutiny
Regulatory expectations can evolve significantly in a short timeframe, driven by tariffs, FDA reforms, and updates from advisory committees such as ACIP.
For pharma teams, September 2025’s headlines highlighted how regulatory developments can escalate quickly into operational and compliance risk. These shifts aren’t anomalies, but reflect an increasingly frequent pattern of regulatory acceleration that pharma teams must be prepared to navigate. Reactive workflows often lead to delayed detection of adverse events, slower responses to public narratives, and limited visibility into compliance breaches. Teams that rely on traditional monitoring or generic alert systems alone often miss fast-moving brand threats, especially during high-volume surges. Without the capacity to surface critical issues in real time, brands face heightened exposure to regulatory penalties and public backlash.
The FDA’s recent wave of enforcement letters, coupled with a renewed push for transparency in drug marketing practices, has raised expectations across the industry. Adverse event detection is no longer a behind-the-scenes function. It’s now a visible, strategic compliance priority for pharmaceutical brands. Teams that treat it as a routine task risk falling behind evolving standards.
You already know how fast things move, but what’s changing now is the regulator’s expectation that you move just as fast. Companies investing in proactive online risk intelligence can adapt more quickly to changing expectations, allowing them to maintain compliance and gain a competitive edge.
How viral claims disrupt pharmacovigilance workflows
The claim linking Tylenol use during pregnancy to autism gained rapid traction online, prompting a surge of public reaction across social media. In response, Tylenol’s official pages disabled comments on newer posts, which had the unintended byproduct of limiting genuine community engagement. Comments on older posts remained open, and were quickly inundated with false claims, brand abuse and criticism, user conflict, and public mockery of the brand’s crisis.
For context: Tylenol’s Instagram posts typically receive fewer than 100 comments. The final post before comments were disabled received over 1,600, highlighting how quickly engagement can spike during periods of heightened public attention.
When pharma-related claims go viral, public perception becomes a risk vector in its own right. These narratives often cross over into owned and paid social channels, far surpassing typical engagement volumes and increasing operational strain.
| Most internal pharmacovigilance teams lack the resources to reliably detect and escalate potential safety signals during high-volume surges. | |
| Without a structured moderation strategy, brand-owned channels can become overwhelmed. The reputational risk is obvious, but so is the exposure to unmonitored adverse event reports. |
In these moments, digital noise shifts from a reputational concern to a pharmacovigilance challenge. When teams can’t isolate actionable concerns from broader conversation trends, both patient safety and regulatory compliance are put at risk.
Why social moderation belongs in your pharmacovigilance strategy
Content moderation isn’t just digital hygiene. It’s a frontline tool for pharmacovigilance and compliance that keeps your community safe and preserves your brand integrity. On both paid and owned pharmaceutical channels, effective, analyst-vetted moderation helps teams identify early safety signals, address reputational threats, and respond to public concerns before they escalate.
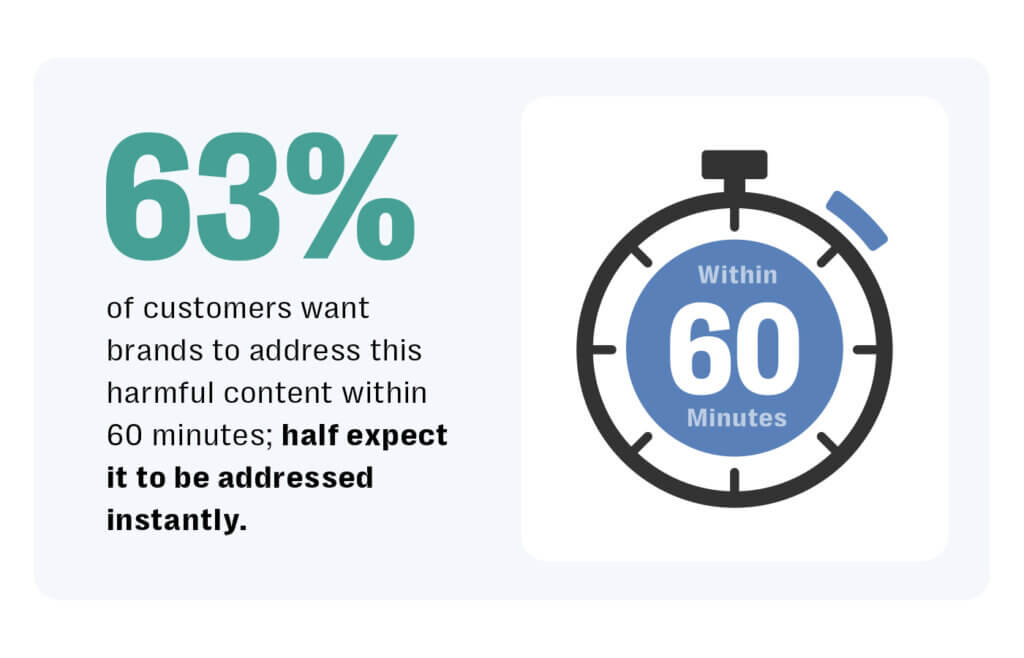
Beyond adverse events, pharma channels are vulnerable to misinformation, off-label promotion, user disputes, and brand impersonation — alongside broader threats like hate speech, scams, and abuse. According to Resolver research, 63% of customers expect brands to address harmful content within 60 minutes; half expect it to be addressed immediately.
Without proper moderation, isolated commentary on owned pages can snowball. A lack of clear escalation protocols increases the risk of missing critical signals embedded in comment threads or community discussions that can escalate into a full-blown brand crisis.
Learn more: Why Social Media Belongs in Your Pharmacovigilance Strategy
Why traditional tools aren’t enough for pharma compliance today
In regulated industries like pharma, standard marketing tools — like keyword-based social listening platforms or general sentiment analysis — often miss the mark when it comes to compliance risk.
|
Sentiment dashboards don’t detect safety signals or noncompliant content, like off-label promotion or adverse event risks |
|
|
Keyword alerts are noisy, reactive, and overlook risks using unanticipated terms or nuanced language |
|
|
Manual review is prone to inconsistency and human error, increasing the chance of missed adverse event signals and regulatory scrutiny |
|
 |
One size doesn’t fit all. When solutions aren’t designed to fit your industry and audience, they miss context, language patterns, and safety triggers unique to pharma |
Without a dedicated analysis team, surges in volume increase brands’ vulnerability, but maintaining surge-ready infrastructure beyond these spikes isn’t cost-effective. Streamlined internal teams can’t guarantee constant multilingual threat and adverse event detection, yet that’s what regulators and customers expect.
Learn more: High-Stakes Influence: How Pharma Marketers Can Partner Without Risk
What 2025 taught us about real-time risk detection
The events of Q3 2025 served as an early warning to the pharmaceutical industry, but brands can take key insights from their impact. On social channels, patients asked whether tariffs would impact their medication prices. Others questioned vaccine eligibility under new ACIP guidance. Following the FDA sent cease-and-desist letters, some users accused implicated brands of misleading consumers, adding to already-prevalent anti-pharmaceutical narratives.
When major regulatory and advisory changes make headlines, patient communities seek guidance, but abusive comments can increase too. Underequipped brands face difficult choices. Some limit volumes and abuse by pausing social media posting or turning off comments, leaving patient communities without support. Others adopt a business-as-usual approach. But without the necessary resources, patient queries and adverse events get lost due to the volume of commentary.
From crisis response to proactive pharma risk intelligence
Forward-looking pharmaceutical teams are using post-event analysis to build scenario playbooks that inform regulatory strategy, adverse event preparedness, public health communication, and investor confidence. In today’s climate, safety signals, misinformation, and regulatory sentiment often emerge through public discourse first. Social media is rife with insights, but most of it gets missed by using legacy dashboards and generic sentiment tracking alone.
Modern social intelligence and pharmacovigilance approaches are proactive and context-aware, providing brands with reassurance and strategic insights. Resolver’s approach combines AI tooling with expert human analysis. We help brands to catch emerging issues that traditional tools might overlook, guarantee 100% adverse event detection, and provide executive-ready insights on complex issues.
 Incidents like the drug tariff announcement, the surge of FDA enforcement letters, and shifts in ACIP guidance all demonstrate how fast online narratives can reshape the regulatory landscape. Integrating learnings from these flashpoints is critical to building a proactive risk strategy.
Incidents like the drug tariff announcement, the surge of FDA enforcement letters, and shifts in ACIP guidance all demonstrate how fast online narratives can reshape the regulatory landscape. Integrating learnings from these flashpoints is critical to building a proactive risk strategy.
Compliance, marketing, and safety teams who invest in this informed, proactive approach gain an advantage over competitors by reducing long-term costs, enhancing patient safety and regulator compliance, and avoiding the risk of disruptive delays.
2025 in hindsight: What smart teams will take into 2026
September was a busy month for pharmaceutical industry headlines, but it wasn’t unique. Pharmaceutical companies face rising scrutiny, ongoing misinformation, and evolving regulatory pressure. With US midterm elections approaching, teams will face even higher stakes in 2026.
Pharma leaders who took recent signals seriously are already making moves:
|
Updating internal escalation pathways for misinformation and AEs |
|
|
Reassessing moderation capacity across owned social channels |
|
 |
Building cross-functional playbooks for when policy meets public discourse |
If your team hasn’t already had these conversations, now is the time.
The cost of missing the signal: A new era for pharma risk leaders
You don’t need to imagine what it looks like when digital narratives turn into compliance problems. 2025’s headlines have given you the case study. Resolver’s dedicated pharmacovigilance adverse event detection and moderation services provide you with visibility of emerging themes and early safety signal distortions, reliable moderation and guaranteed 100% adverse event detection through unexpected volume surges, and confidence to keep connecting with your community through crises.
See how your team can detect pharma compliance risks earlier and act faster: Talk to an expert
About the author: Megan Ardis is a Specialist Analyst for Resolver’s Corporate Intelligence division, where she helps clients navigate fast-moving reputational and regulatory risks. With a background in microbiology and crisis intelligence, she brings deep insight into the intersection of public health, misinformation, and online risk. Before joining Resolver, Megan worked as an editor and writer, covering science, policy, and digital trends. She holds a master’s degree in History of Science, Technology and Medicine from the University of Leeds.

